IDRA-21
化合物
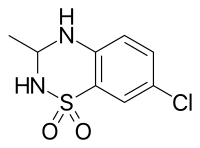
IDRA-21是種AMPA受體的正別構調節劑,也是苯丙噻二嗪的衍生物,也是一種手性分子,(+)-IDRA-21是其活性形式。[1]
 | |
| 法律規範狀態 | |
|---|---|
| 法律規範 |
|
| 識別資訊 | |
| |
| CAS號 | 22503-72-6 |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化學資訊 | |
| 化學式 | C8H9ClN2O2S |
| 摩爾質量 | 232.68 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
在動物實驗中,IDRA-21表現出了有促進學習的效果,並能明顯改善學習和記憶。在逆轉阿普唑侖或東莨菪鹼誘發的認知障礙方面,有着約比茴拉西坦10到30倍的效力,[2][3]且在單次服用後,可產生長達48小時的持續效應,[4]其作用機制,被認為是通過促進大腦突觸間的誘導LTP達到的。[5]
正常情況下,IDRA-21或許不產生神經毒性,[6]但可能會加重中風或癲癇發作後,因全身缺血所造成的神經元損傷。[7]
與安帕金或苯甲酰哌啶衍生的AMPA受體增效劑相比,IDRA-21藥效優於CX-516,低於CX-546。[8]與IDRA-21相比,效力更好的苯丙噻二嗪類衍生物已被開發出來,[9][10]但這些衍生物,各所獲的研究程度並不一樣,苯甲酰基哌啶(benzoylpiperidine)和苯甲酰基吡咯烷CX系列(benzoylpyrrolidine CX-series)藥物,在臨床開發中更受青睞,這或是由於它們在高劑量使用時,具有更有利的毒性特徵。[11]
另見
編輯參考文獻
編輯- ^ Uzunov DP, Zivkovich I, Pirkle WH, Costa E, Guidotti A. Enantiomeric resolution with a new chiral stationary phase of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide, a cognition-enhancing benzothiadiazine derivative. Journal of Pharmaceutical Sciences. August 1995, 84 (8): 937–42. PMID 7500277. doi:10.1002/jps.2600840807.
- ^ Thompson DM, Guidotti A, DiBella M, Costa E. 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys. Proceedings of the National Academy of Sciences of the United States of America. August 1995, 92 (17): 7667–71. Bibcode:1995PNAS...92.7667T. PMC 41206 . PMID 7644474. doi:10.1073/pnas.92.17.7667 .
- ^ Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A. 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. The Journal of Pharmacology and Experimental Therapeutics. January 1995, 272 (1): 300–9. PMID 7815345.
- ^ Buccafusco JJ, Weiser T, Winter K, Klinder K, Terry AV. The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys. Neuropharmacology. January 2004, 46 (1): 10–22. PMID 14654093. S2CID 26443642. doi:10.1016/j.neuropharm.2003.07.002.
- ^ Arai A, Guidotti A, Costa E, Lynch G. Effect of the AMPA receptor modulator IDRA 21 on LTP in hippocampal slices. NeuroReport. September 1996, 7 (13): 2211–5. PMID 8930991. S2CID 35888339. doi:10.1097/00001756-199609020-00031.
- ^ Impagnatiello F, Oberto A, Longone P, Costa E, Guidotti A. 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide: a partial modulator of AMPA receptor desensitization devoid of neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. June 1997, 94 (13): 7053–8. Bibcode:1997PNAS...94.7053I. PMC 21283 . PMID 9192690. doi:10.1073/pnas.94.13.7053 .
- ^ Yamada KA, Covey DF, Hsu CY, Hu R, Hu Y, He YY. The diazoxide derivative IDRA 21 enhances ischemic hippocampal neuron injury. Annals of Neurology. May 1998, 43 (5): 664–9. PMID 9585363. S2CID 39977647. doi:10.1002/ana.410430517.
- ^ Nagarajan N, Quast C, Boxall AR, Shahid M, Rosenmund C. Mechanism and impact of allosteric AMPA receptor modulation by the ampakine CX546. Neuropharmacology. November 2001, 41 (6): 650–63. PMID 11640919. S2CID 7796112. doi:10.1016/S0028-3908(01)00133-2.
- ^ Phillips D, Sonnenberg J, Arai AC, Vaswani R, Krutzik PO, Kleisli T, et al. 5'-alkyl-benzothiadiazides: a new subgroup of AMPA receptor modulators with improved affinity. Bioorganic & Medicinal Chemistry. May 2002, 10 (5): 1229–48. CiteSeerX 10.1.1.113.7845 . PMID 11886787. doi:10.1016/S0968-0896(01)00405-9.
- ^ Arai AC, Xia YF, Kessler M, Phillips D, Chamberlin R, Granger R, Lynch G. Effects of 5'-alkyl-benzothiadiazides on (R,S)-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor biophysics and synaptic responses. Molecular Pharmacology. September 2002, 62 (3): 566–77. PMID 12181433. S2CID 16182942. doi:10.1124/mol.62.3.566.
- ^ Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology. April 2005, 179 (1): 154–63. PMID 15672275. S2CID 5869366. doi:10.1007/s00213-004-2065-6.