達爾奈多
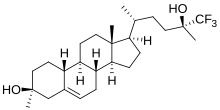
達爾奈多(INN:dalzanemdor;開發代號:SAGE-718)是一種正在研究用於治療神經系統疾病和認知障礙的實驗性藥物,[1]是神經甾體腦甾醇的類似物。[2]截至2022年,它在阿茲海默症、[3][4][5]柏金遜症和亨丁頓舞蹈症[6][7]的治療上處於二期臨床研究階段。
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | SAGE-718 |
| 法律規範狀態 | |
| 法律規範 |
|
| 識別資訊 | |
| |
| CAS號 | 2311911-06-3 |
| PubChem CID | |
| KEGG | |
| ChEMBL | |
| 化學資訊 | |
| 化學式 | C26H41F3O2 |
| 摩爾質量 | 442.61 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
參考文獻
編輯- ^ Hill MD, Blanco MJ, Salituro FG, Bai Z, Beckley JT, Ackley MA, et al. SAGE-718: A First-in-Class N-Methyl-d-Aspartate Receptor Positive Allosteric Modulator for the Potential Treatment of Cognitive Impairment. Journal of Medicinal Chemistry. July 2022, 65 (13): 9063–9075. PMID 35785990. S2CID 250250073. doi:10.1021/acs.jmedchem.2c00313.
- ^ SAGE-718. ALZFORUM. [2024-09-11]. (原始內容存檔於2023-12-20).
- ^ Shapiro L. #AAN2022 – SAGE-718 May Help With Cognitive Function in Alzheimer's. BioNews, Inc. 2022-04-05 [2024-09-11]. (原始內容存檔於2023-12-20).
- ^ SAGE-718 in Patients With Mild Cognitive Impairment or Mild Dementia Due to Alzheimer's Disease: Results From the Phase 2 LUMINARY Study (PDF). American Academy of Neurology (AAN) 74th Annual Meeting Abstract. [2024-09-11]. (原始內容存檔於2022-04-05).
- ^ Castañeda R. Regulatory roundup: Sage Alzheimer's asset likely to progress after Phase IIa completion. Clinical Trials Arena. 2021-11-16 [2022-07-13]. (原始內容存檔於2022-05-28).
- ^ Sage Therapeutics Provides Important Update On The Clinical Development Program For Sage's Investigational Drug, SAGE-718.. Huntington's Disease Society of America. 2022-02-22 [2024-09-11]. (原始內容存檔於2024-12-02).
- ^ Wexler M. SAGE-718 on FDA Fast Track as Potential Huntington's Disease Therapy. BioNews, Inc. 2021-09-21 [2024-09-11]. (原始內容存檔於2024-11-30).
外部連結
編輯| 這是一篇作用於神經系統的藥品小作品。您可以透過編輯或修訂擴充其內容。 |