非布司他
非布司他(Febuxostat)是一种用于治疗痛风的药物,而藥品所針對的痛風基本因尿酸水平过高而引起[4]。該藥物通過抑制黄嘌呤氧化酶,从而减少体内尿酸的产生。該藥的給藥途徑為口服。該藥常见的副作用包括肝脏出現问题、恶心、关节痛和皮疹[4]等症狀。妊娠或哺乳期婦女不建议服用該藥物[5][6]。
 | |
| 臨床資料 | |
|---|---|
| 商品名 | Uloric, Adenuric[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609020 |
| 核准狀況 |
|
| 懷孕分級 | |
| 给药途径 | 口服 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | ≥84% absorbed |
| 血漿蛋白結合率 | 99.2% to albumin |
| 药物代谢 | 通過CYP1A1, CYP1A2, CYP2C8, CYP2C9, UGT1A1, UGT1A8, UGT1A9[3] |
| 生物半衰期 | ~5–8 小時 |
| 排泄途徑 | Urine (~49%, mostly as metabolites, 3% as unchanged drug); feces (~45%, mostly as metabolites, 12% as unchanged drug) |
| 识别信息 | |
| |
| CAS号 | 144060-53-7 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.329 |
| 化学信息 | |
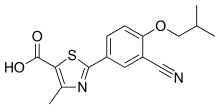
| 化学式 | C16H16N2O3S |
| 摩尔质量 | 316.38 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
非布司他于2008年和2009年分别在欧盟和美国被批准用于医疗用途[7][4]。2019年,该藥的仿制藥被監管部門批准,2020年起正式上市[8] [9]。
参考文献
编辑- ^ Drugs.com Drugs.com international names for febuxostat (页面存档备份,存于互联网档案馆) Page accessed June 25, 2015
- ^ 2.0 2.1 Febuxostat (Uloric) Use During Pregnancy. Drugs.com. 22 February 2019 [17 May 2020]. (原始内容存档于2021-02-09).
- ^ Adenuric: EPAR – Product Information (PDF). European Medicines Agency. 2019-08-06 [2021-02-04]. (原始内容 (PDF)存档于2021-08-28).
- ^ 4.0 4.1 4.2 Febuxostat Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [26 February 2019]. (原始内容存档于2021-02-08) (英语).
- ^ British national formulary : BNF 76 76. Pharmaceutical Press. 2018: 1087. ISBN 9780857113382.
- ^ Drug Safety and Availability - FDA adds Boxed Warning for increased risk of death with gout medicine Uloric (febuxostat). FDA. 21 February 2019 [26 February 2019]. (原始内容存档于2019-04-23) (英语).
- ^ Adenuric. European Medicines Agency - Commission. 17 September 2018 [26 February 2019]. (原始内容存档于2020-12-30) (英语).
- ^ Generic Uloric Availability. Drugs.com. [1 August 2019]. (原始内容存档于2020-11-11) (英语).
- ^ Febuxostat Generic Uloric. [15 April 2020]. (原始内容存档于2016-10-16).